Molecular radiation biology
The search for radiation-triggered molecular mechanisms to develop bench-to-bedside personalized treatment strategies

Research
The primary goal of radiation therapy is to kill tumor cells. Radiation modulates a plethora of cellular signaling mechanisms that could be exploited for therapeutic benefit. Our main research focus is radiation-induced anti-tumor immunogenicity using conventional photons as well as ions at MedAustron – a unique Austrian center for Ion Therapy and Research.
Recent research showed that radiation in combination with immune checkpoint blockade can clear cancer organism-wide, even in advanced metastasized disease. The success of this approach is, however, limited to a subset of cancers and the molecular mechanisms are poorly understood. We aim at pinpointing the molecular mechanisms necessary to kickstart immune system-mediated tumor clearing in those cancers that have not yet benefitted from immunotherapy. Can we identify specific mutations involved in suppression of anti-tumor immunogenicity? Which molecular mechanisms are involved? Is there a link between radiation-induced anti-tumor immunogenicity and DNA repair? Does autophagy play a role? Is tumor neoantigen presentation influenced by the type of radiation? Can we develop treatment strategies based on our findings?
We use a variety of standard as well as advanced molecular biology techniques. Immune-evasion is tightly linked to the tumor microenvironment. To address this important key aspect, we use heterotypic 3D tumor models, consisting of tumor as well as stromal components. Our future work will focus on the establishment of in vivo models and patient liquid biopsies in collaboration with the medical team on site.
Interferon Type 1 – anti-tumor immunogenicity master regulator
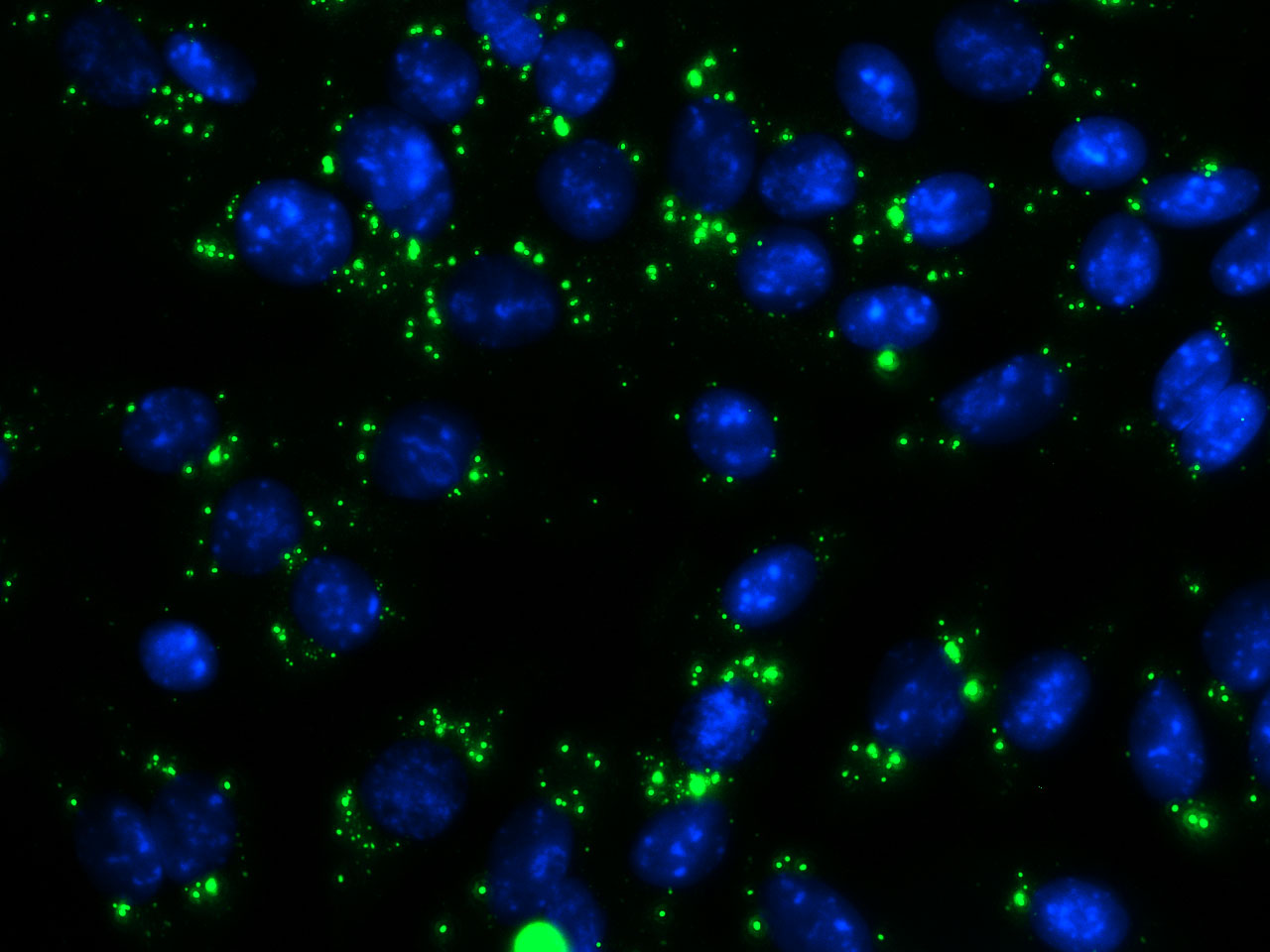
Interferon 1β is the key component of successful anti-tumor immunogenicity activation. Our results show that conventional photons or protons stimulate IFN1β only after fractionated radiation regimen, but not after single dose treatment. Our future aim is to investigate whether physically heavier and hence more biologically effective carbon ions can activate IFN1β. In addition, combination treatment with DNA repair inhibitors will be used to induce synthetic lethality.
Dysfunctional STING – a pancreatic cancer villain
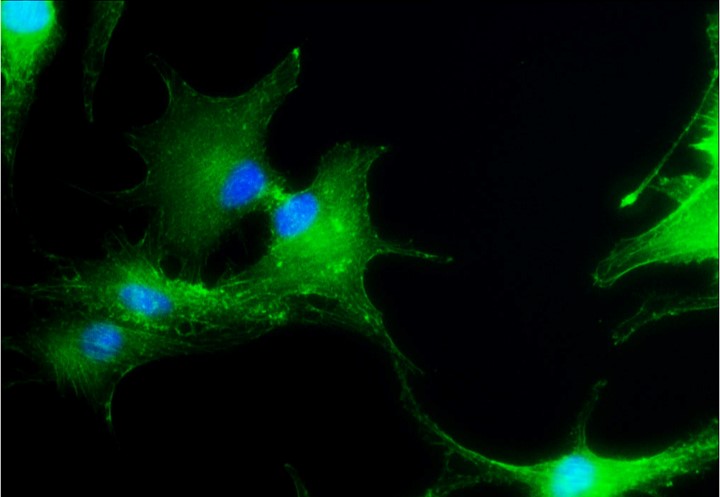
Anti-tumor immunogenicity is based on STING-dependent interferon type 1 activation. Many cancers harbor STING mutations, contributing to the immunosuppressive phenotype of most tumors. Radiation alone fails to activate STING in pancreatic cancer cells. Our aim is to identify and employ STING agonists to induce synthetic lethality in combination with radiation.
Hypoxia – shaping an immunosuppressive tumor microenvironment
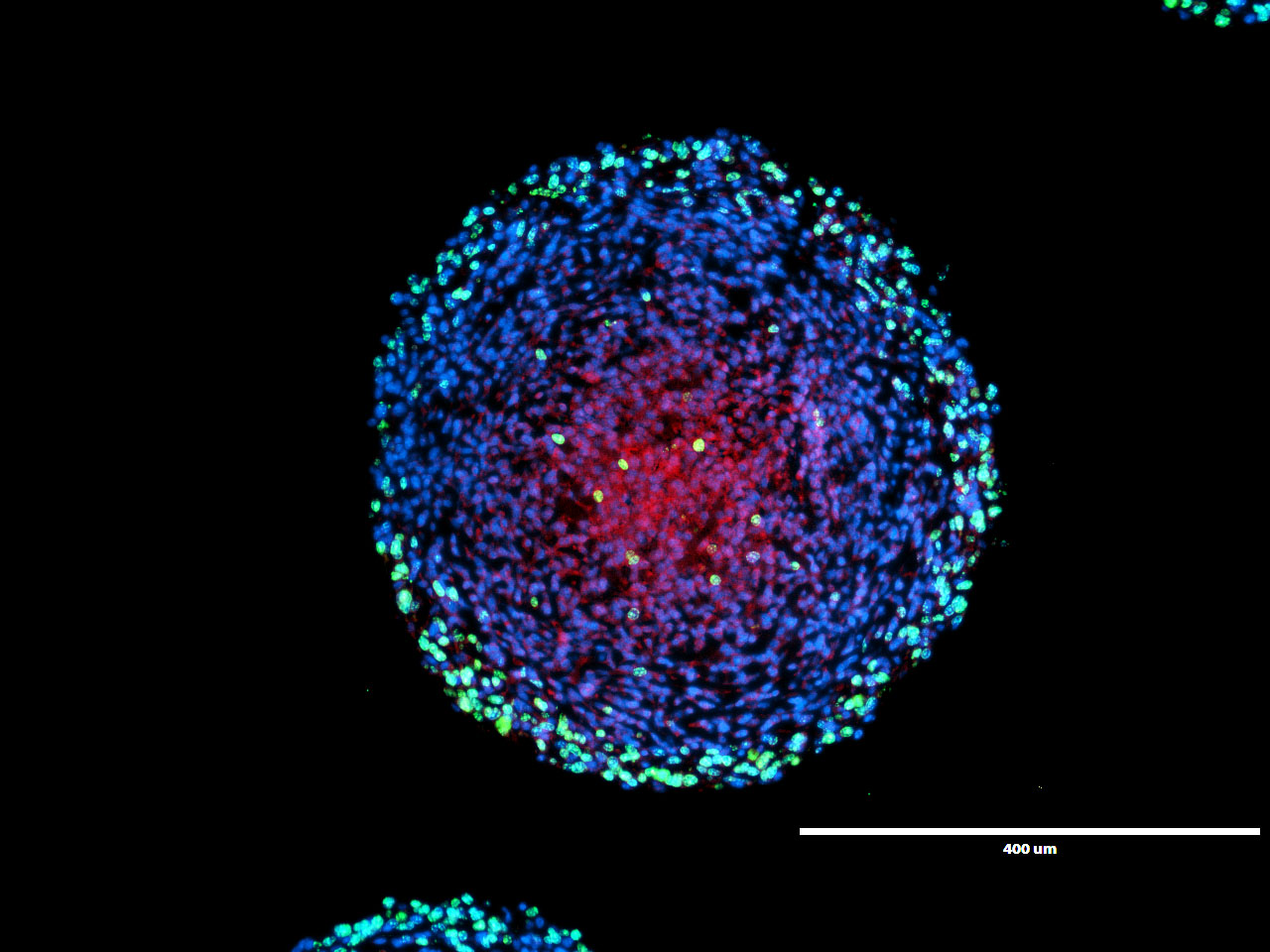
We have identified hypoxia as a key immunosuppressive factor. We showed that the hypoxic tumor spheroid areas are not quiescent but rather actively express immunosuppressive proteins while suppressing immunogenic tumor microenvironment key players. The impact of hypoxia on radiation-induced anti-tumor immunogenicity is investigated in hypoxic spheroid models.
Selected publications
Far far away, behind the word mountains, far from the countries Vokalia and Consonantia, there live the blind texts.
The interplay between autophagy and cGAS-STING signaling and its implications for cancer, Front Immunol 2024
Effects of a combined therapy of bortezomib and ionizing radiation on chondrosarcoma three-dimensional spheroid cultures, Oncol Letter 2021
CD73 Blockade Promotes Dendritic Cell Infiltration of Irradiated Tumors and Tumor Rejection, Cancer Immunol Res 2020
PARP and PARG inhibitors in cancer treatment, Genes Dev 2020
Phantom design and dosimetric characterization for multiple simultaneous cell irradiations with active pencil beam scanning, Radiat Environ Biophys 2019
Radioprotective Effects of Dermatan Sulfate in a Preclinical Model of Oral Mucositis-Targeting Inflammation, Hypoxia and Junction Proteins without Stimulating Proliferation, Int J Mol Sci 2018
Upregulated epithelial junction expression represents a novel parameter of the epithelial radiation response to fractionated irradiation in oral mucosa, Strahlenther Onkol 2018
Protective effects of systemic dermatan sulfate treatment in a preclinical model of radiation-induced oral mucositis, Strahlenther Onkol 2018
PARP inhibition causes premature loss of cohesion in cancer cells, Oncotarget 2017
Team

Sylvia Kerschbaum-Gruber, PhD
Medical University of Vienna
PostDoc

Niccolo Bragato, MSc
Medical University of Vienna
Technical assistant

Alexandra Kneringer, BSc
University of Vienna
Master student

Ana Beatriz Dias, Msc
Medical University of Vienna
PhD student

Dea Slade, PhD
Medical University of Vienna
Principle investigator

Patrick Fischer, MSc
MedAustron
Research associate

Anna Röhrer, MSc
Medical University of Vienna
Technical assistant

Elisabeth Mara, BSc
FH Wiener Neustadt
Collaborator

Collaborations
MedAustron
Ion Therapy and Research Center
Wiener Neustadt, AUSTRIA
Sandra DEMARIA
Weill Cornell Medicine
New York, USA
Birgit LOHBERGER
Medical University of Graz
Graz, AUSTRIA
Martin PRUSCHY
University Hospital Zürich
Zürich, SWITZERLAND
FH Wiener Neustadt
Fachhochschule
Wiener Neustadt, AUSTRIA
Contact
Medical University of Vienna
Department of Radiation Oncology
Währinger Gürtel 18-20
1090 Vienna AUSTRIA
MedAustron
Marie-Curie Straße 5
2700 Wiener Neustadt AUSTRIA